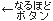IDE

investigational device exemption (protocol)
(アメリカの食品医薬品局の)研究装置免除(制度).
investigational device exemption (protocol); by the FDA(Food and Drug Administration); a medical exemption protocol for new trial devices; ref. PMA(premarket-approval application) by FDA; (US);
[Med:Pol].

 | 丸善 「略語大辞典」 JLogosID : 11878021 |